Theoretical Electrochemical Study and Calculation of Free Energies of Electron Transfer in B-Cyclodextrins/Fullerenes C60 Nanostructure Complexes
DOI:
https://doi.org/10.61186/jcc.5.1.3Keywords:
Fullerenes, β–Cyclodextrins, The electron transfer energies, Rate constants, Marcus theoryAbstract
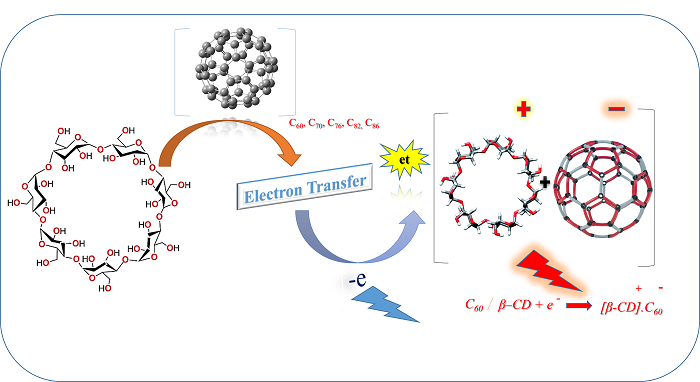
Cyclodextrin is a cyclic molecule that contains the three essential six, seven, and eight glucose molecules, called by the names a, B, and y-cyclodextrin, individually. Cyclodextrins compounds are thought to be completely polar as a result of the hydroxyl groups present in glucose moieties. Secondary C2-hydroxyl groups of glucose units are found on the secondary face, while the C6-hydroxyl type groups are located on the primary face because these are related to the primary face of the incomplete cone. The C1 group, a glucoside oxygen ring, and another ring of C-H groups make up the inside of the cyclodextrin cone, making it rather nonpolar. Hydrophobic fullerenes compounds Cn [n= 60, 70, 76, 82, and 86] have been chosen for the guest molecules and different parameters like first to fourth free activations, the kinetic rate constant, the energies of electron transfer (ket(n)), and ?G#et(n) where (n=1-4), were calculated and discussed in detail. All the computed results showed the best coherence with the Marcus theory. Different analyses suggested that free energy is lowered due to an efficient electron transfer, which begins with the first step (the first of the four activate free energy values).
References
H. Sahrayi, E. Hosseini, S. Karimifard, N. Khayam, S.M. Meybodi, S. Amiri, M. Bourbour, B. Farasati Far, I. Akbarzadeh, M. Bhia, C. Hoskins, C. Chaiyasut, Co-Delivery of Letrozole and Cyclophosphamide via Folic Acid-Decorated Nanoniosomes for Breast Cancer Therapy: Synergic Effect, Augmentation of Cytotoxicity, and Apoptosis Gene Expression, Pharmaceuticals, 2022.
C. Muankaew, T. Loftsson, Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery, Basic and, Clinical Pharmacology and, Toxicology 122(1) (2018) 46-55.
F. Eshrati Yeganeh, A. Eshrati Yeganeh, M. Fatemizadeh, B. Farasati Far, S. Quazi, M. Safdar, In vitro cytotoxicity and anti-cancer drug release behavior of methionine-coated magnetite nanoparticles as carriers, Medical Oncology 39(12) (2022) 252.
B. Farasati Far, S. Asadi, M.R. Naimi-Jamal, W.K. Abdelbasset, A. Aghajani Shahrivar, Insights into the interaction of azinphos-methyl with bovine serum albumin: experimental and molecular docking studies, Journal of Biomolecular Structure and Dynamics 40(22) (2022) 11863-11873.
M.A. Przybyla, G. Yilmaz, C.R. Becer, Natural cyclodextrins and their derivatives for polymer synthesis, Polymer Chemistry 11(48) (2020) 7582-7602.
S. Choudhary, L. Gupta, S. Rani, K. Dave, U. Gupta, Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules, 8 (2017).
A. Celebioglu, T. Uyar, Hydrocortisone/cyclodextrin complex electrospun nanofibers for a fast-dissolving oral drug delivery system, RSC Medicinal Chemistry 11(2) (2020) 245-258.
Y.-A. Choi, B.R. Chin, D.H. Rhee, H.-G. Choi, H.-W. Chang, J.-H. Kim, S.-H. Baek, Methyl-B-cyclodextrin inhibits cell growth and cell cycle arrest via a prostaglandin E(2) independent pathway, Experimental and, Molecular Medicine 36(1) (2004) 78-84.
A. Stasilowicz, E. Tykarska, N. Rosiak, K. Salat, A. Furgala-Wojas, T. Plech, K. Lewandowska, K. Pikosz, K. Pawlowicz, J. Cielecka-Piontek, The Inclusion of Tolfenamic Acid into Cyclodextrins Stimulated by Microenvironmental pH Modification as a Way to Increase the Anti-Migraine Effect, Journal of Pain Research 14 (2021) 981-992.
P. Berben, J. Stappaerts, M.J.A. Vink, E. Dominguez-Vega, G.W. Somsen, J. Brouwers, P. Augustijns, Linking the concentrations of itraconazole and 2-hydroxypropyl-B-cyclodextrin in human intestinal fluids after oral intake of Sporanox, European Journal of Pharmaceutics and Biopharmaceutics 132 (2018) 231-236.
Y. Xu, C. Zhang, X. Zhu, X. Wang, H. Wang, G. Hu, Q. Fu, Z. He, Chloramphenicol/sulfobutyl ether-B-cyclodextrin complexes in an ophthalmic delivery system: prolonged residence time and enhanced bioavailability in the conjunctival sac, Expert Opinion on Drug Delivery 16(6) (2019) 657-666.
S.S. Braga, Cyclodextrins: Emerging Medicines of the New Millennium, Biomolecules, 2019.
M.H. Asim, M. Ijaz, A. Mahmood, P. Knoll, A. Jalil, S. Arshad, A. Bernkop-Schnürch, Thiolated cyclodextrins: Mucoadhesive and permeation enhancing excipients for ocular drug delivery, International Journal of Pharmaceutics 599 (2021) 120451.
P.C.S. Costa, J.S. Evangelista, I. Leal, P.C.M.L. Miranda, Chemical graph theory for property modeling in QSAR and QSPR—charming QSAR and, QSPR, Mathematics 9(1) (2020) 60.
Z. Foroutan, A.R. Afshari, Z. Sabouri, A. Mostafapour, B.F. Far, M. Jalili-Nik, M. Darroudi, Plant-based synthesis of cerium oxide nanoparticles as a drug delivery system in improving the anticancer effects of free temozolomide in glioblastoma (U87) cells, Ceramics International 48(20) (2022) 30441-30450.
F. Eshrati Yeganeh, A. Eshrati Yeganeh, M. Fatemizadeh, B. Farasati Far, S. Quazi, M.J.M.O. Safdar, In vitro cytotoxicity and anti-cancer drug release behavior of methionine-coated magnetite nanoparticles as carriers, 39(12) (2022) 252.
F. Ban, K. Dalal, H. Li, E. LeBlanc, P.S. Rennie, A. Cherkasov, Best Practices of Computer-Aided Drug Discovery: Lessons Learned from the Development of a Preclinical Candidate for Prostate Cancer with a New Mechanism of Action, Journal of Chemical Information and Modeling 57(5) (2017) 1018-1028.
K. Elaheh, G. Nosrat, Potential applications of nanoshell bow-tie antennas for biological imaging and hyperthermia therapy, Optical Engineering 58(6) (2019) 065102.
I. Hdoufane, D. Ounaissi, A. Dermoune, D. Cherqaoui, Development of QSAR Models Using Singular Value Decomposition Method: A Case Study for Predicting Anti-HIV-1 and Anti-HCV Biological Activities, Biointerface Research in Applied Chemistry 12(3) (2021) 3090-3105.
H. Shen, The compressive mechanical properties of Cn (n=20, 60, 80, 180) and endohedral M@C60 (M=Na, Al, Fe) fullerene molecules, Molecular Physics 105(17-18) (2007) 2405-2409.
F. Lu, E.A. Neal, T. Nakanishi, Self-Assembled and Nonassembled Alkylated-Fullerene Materials, Accounts of Chemical Research 52(7) (2019) 1834-1843.
L. Kavan, L. Dunsch, H. Kataura, Electrochemical tuning of electronic structure of carbon nanotubes and fullerene peapods, Carbon 42(5) (2004) 1011-1019.
N. Kamanina, Fullerenes and Relative Materials: Properties and Applications, IntechOpen2018.
R.E. Haufler, J. Conceicao, L.P.F. Chibante, Y. Chai, N.E. Byrne, S. Flanagan, M.M. Haley, S.C. O’Brien, C. Pan, et al., Efficient production of C60 (buckminsterfullerene), C60H36, and the solvated buckide ion, The Journal of Physical Chemistry 94(24) (1990) 8634-8636.
P.A. Maggard, X. Cheng, S. Deng, M.-H. Whangbo, Physical Properties of Molecules and Condensed Materials Governed by Onsite Repulsion, Spin-Orbit Coupling and Polarizability of Their Constituent Atoms, Molecules 25(4) (2020) 867.
R. Otero, A.L. Vázquez de Parga, J.M. Gallego, Electronic, structural and chemical effects of charge-transfer at organic/inorganic interfaces, Surface Science Reports 72(3) (2017) 105-145.
R. Purchase, H.J.I.F. De Groot, Biosolar cells: global artificial photosynthesis needs responsive matrices with quantum coherent kinetic control for high yield, 5(3) (2015) 20150014.
M. Najafpour, Applied Photosynthesis: New Progress, IntechOpen2016.
S.-J. Huang, Y.-T. Hsu, Faithful derivation of symmetry indicators: A case study for topological superconductors with time-reversal and inversion symmetries, Physical Review Research 3(1) (2021) 013243.
X. Chen, L. Chen, S. Zheng, H. Wang, Y. Dai, Z. Chen, R. Huang, Disrupted Brain Connectivity Networks in Aphasia Revealed by Resting-State fMRI, 13 (2021).
L. Wang, X. Xue, X. Zhou, Z. Wang, R. Liu, Analyzing the topology characteristic and effectiveness of the China city network, Environment and Planning B: Urban Analytics and City Science 48(9) (2021) 2554-2573.
A.A. Taherpour, D. Narian, A. Taherpour, Structural relationships and theoretical study of the free energies of electron transfer, electrochemical properties, and electron transfer kinetic of cephalosporin antibiotics derivatives with fullerenes in nanostructure of [R]·Cn(R = cefadroxil, cefepime, cephalexin, cefotaxime, cefoperazone and ceftriaxone) supramolecular complexes, Journal of Nanostructure in Chemistry 5(2) (2015) 153-167.
A. Taherpour, M. Maleki-Noureini, Free Energies of Electron Transfer, Electron Transfer Kinetic Theoretical and Quantitative Structural Relationships and Electrochemical Properties Studies of Gadolinium Nitride Cluster Fullerenes Gd3N@Cn in [X-UT-Y][Gd3N@Cn](n = 80, 82, 84, 86 and 88) Supramolecular Complexes, Fullerenes, Nanotubes and Carbon Nanostructures 21(6) (2013) 485- 502.
H.R.A. El-Mageed, F.M. Mustafa, M.K. Abdel-Latif, Boron nitride nanoclusters, nanoparticles and nanotubes as a drug carrier for isoniazid anti-tuberculosis drug, computational chemistry approaches, Journal of Biomolecular Structure and Dynamics 40(1) (2022) 226-235.
B. Farasati Far, M.R. Naimi-Jamal, M. Jahanbakhshi, H.T. Mohammed, U.S. Altimari, J. Ansari, Poly(3-thienylboronic acid) coated magnetic nanoparticles as a magnetic solid-phase adsorbent for extraction of methamphetamine from urine samples, Journal of Dispersion Science and Technology (2022) 1-11.
B. Farasati Far, D. Bokov, G. Widjaja, H. Setia Budi, W. Kamal Abdelbasset, S. Javanshir, F. Seif, H. Pazoki-Toroudi, S.K. Dey, Metronidazole, acyclovir and tetrahydrobiopterin may be promising to treat COVID-19 patients, through interaction with interleukin-12, Journal of Biomolecular Structure and Dynamics (2022) 1-19.
C.A. Celaya, J. Muniz, L.E. Sansores, New nanostructures of carbon: Quasi-fullerenes Cn-q (n=20, 42, 48, 60), Computational and Theoretical Chemistry 1117 (2017) 20-29.
C. Bannwarth, S. Ehlert, S. Grimme, GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions, Journal of Chemical Theory and Computation 15(3) (2019) 1652-1671.
R.A.J.T.J.o.c.p. Marcus, On the theory of oxidation-reduction reactions involving electron transfer. I, 24(5) (1956) 966-978.
A.T. Avat, A.-R. Shafaati, Theoretical Study of Structural Relationships and Electrochemical Properties of [DNA-Nucleotide Bases]@ Cn Complexes, Oriental Journal of Chemistry 27(3) (2011) 823.
B. Farasati Far, K. Vakili, M. Fathi, S. Yaghoobpoor, M. Bhia, M.R. NaimiJamal, The role of microRNA-21 (miR-21) in pathogenesis, diagnosis, and prognosis of gastrointestinal cancers: A review, Life Sciences 316 (2023) 121340.
B. Farasati Far, S. Asadi, M.R. Naimi-Jamal, W.K. Abdelbasset, A.J.J.o.B.S. Aghajani Shahrivar, Dynamics, Insights into the interaction of azinphos-methyl with bovine serum albumin: Experimental and molecular docking studies, 40(22) (2022) 11863-11873.
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 The University of Georgia Publishing House (UGPH)

This work is licensed under a Creative Commons Attribution 4.0 International License.

