Poly (d/l) lactide-polycaprolactone/bioactive glass nanocomposites: assessments of in vitro bioactivity and biodegradability
DOI:
https://doi.org/10.52547/jcc.3.4.1Keywords:
In-vitro test, PDLLA/PCL, Bioactive glass nanoparticles, Bioactivity and BiodegradationAbstract
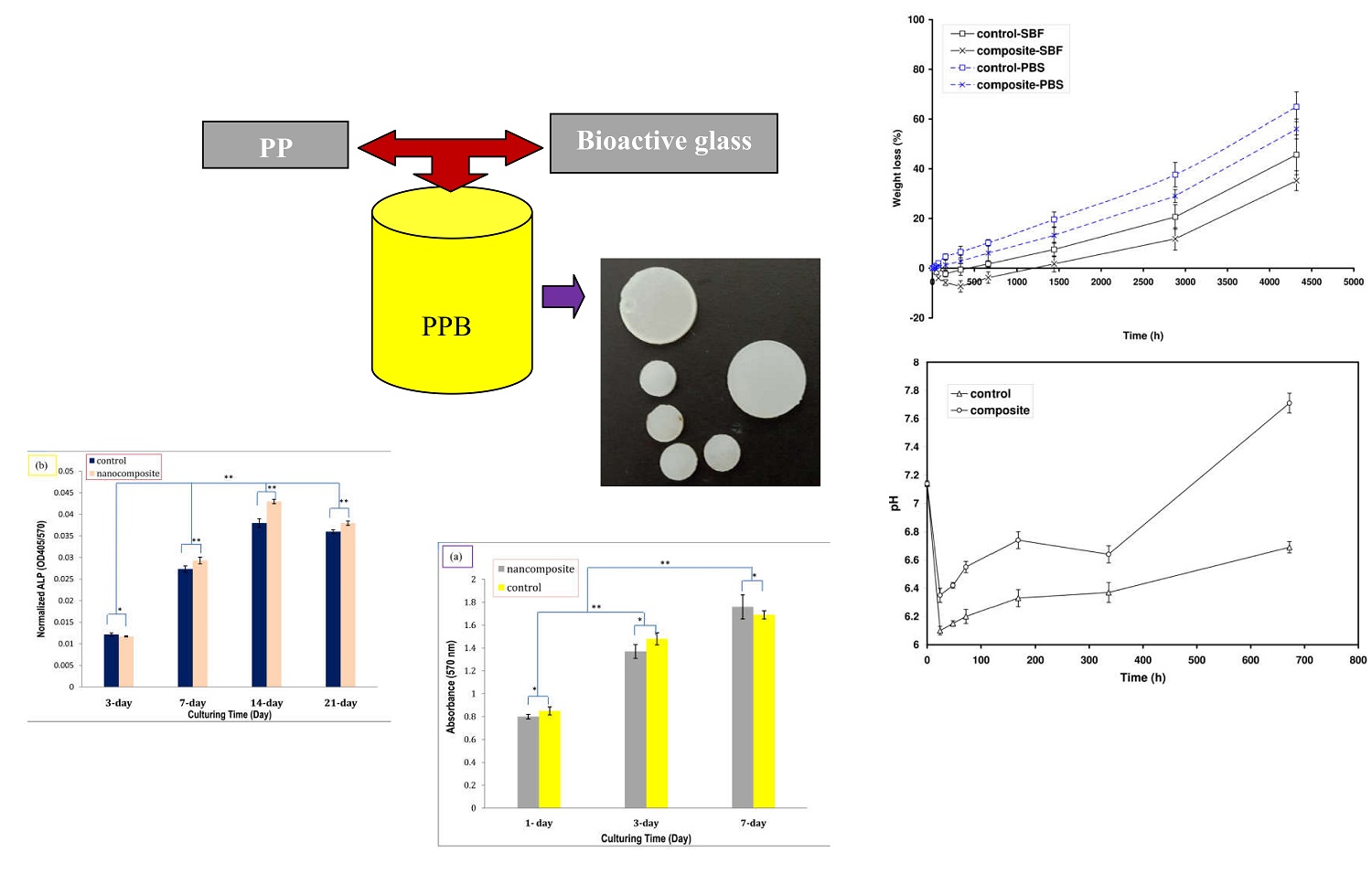
The in vitro assessments suggested the essential features for the bio screws applications. The effects of bioactive glass nanoparticles (BG) during in vitro studies of the poly (D/L) lactide (PDLLA)/polycaprolactone (PCL)/BG nanocomposites (PPB) were assessed. The PDLLA/PCL (PP) blends were chosen as control groups. Apatite formations capabilities, weight and pH variations, alkaline phosphatase activity (ALP), and MTT assay were assigned during different immersion times up to 6 months. The XRD and SEM results revealed the superior apatite formation of PPB in simulated body fluids (SBF) compared to PP. The weight loss and pH variation results illustrated the highest values related to PP. Moreover, the MG-63 cells cultures determined the better cell viability of the PPB compared to the PP blends. Although, there are no statistically significant differences between the two groups. In addition, similar trends are shown for the ALP results where these amounts after 2 and 3 weeks incubation are considerable for PPB in comparison to PP. However, there are also no statistically significant differences between the two groups. Overall, the in vitro bioactivity and biodegradability confirmed that the PPB implants can be promised as a proper candidate for anterior cruciate ligament reconstruction screws.
References
T. Zantop, A. Weimann, R. Schmidtko, M. Herbort, M.J. Raschke, W. Petersen, Graft laceration and pullout strength of soft-tissue anterior cruciate ligament reconstruction: in vitro study comparing titanium, poly-d, l-lactide, and poly-d, l-lactide–tricalcium phosphate screws, Arthroscopy: The Journal of Arthroscopic & Related Surgery 22(11) (2006) 1204-1210.
R. Papalia, S. Vasta, S. D’Adamio, A. Giacalone, N. Maffulli, V. Denaro, Metallic or bioabsorbable interference screw for graft fixation in anterior cruciate liga-ment (ACL) reconstruction?, British medical bulletin 109(1) (2014) 19-29.
P. Debieux, C.E. Franciozi, M. Lenza, M.J. Tamaoki, R.A. Magnussen, F. Faloppa, J.C. Belloti, Bioabsorbable versus metallic interference screws for graft fixation in anterior cruciate ligament reconstruction, Cochrane Database of Systematic Reviews (7) (2016).
A.-S. Moisala, T. Järvelä, A. Paakkala, T. Paakkala, P. Kannus, M. Järvinen, Comparison of the bioabsorbable and metal screw fixation after ACL reconstruction with a hamstring autograft in MRI and clinical outcome: a prospective randomized study, Knee Surgery, Sports Traumatology, Arthroscopy 16 (2008) 1080-1086.
C. Kaeding, J. Farr, T. Kavanaugh, A. Pedroza, A prospective randomized comparison of bioabsorbable and titanium anterior cruciate ligament interference screws, Arthroscopy: The Journal of Arthroscopic & Related Surgery 21(2) (2005) 147-151.
F.D. Bach, R.Y. Carlier, J.B. Elis, D.M. Mompoint, A. Feydy, O. Judet, P. Beaufils, C. Vallée, Anterior cruciate ligament reconstruction with bioabsorbable poly-glycolic acid interference screws: MR imaging follow-up, Radiology 225(2) (2002) 541-550.
J.C. Middleton, A.J. Tipton, Synthetic biodegradable polymers as orthopedic devices, Biomaterials 21(23) (2000) 2335-2346.
J. Esmaeilzadeh, S. Hesaraki, S.M.-M. Hadavi, M.H. Ebrahimzadeh, M. Esfandeh, Poly (d/l) lactide/polycaprolactone/bioactive glasss nanocomposites materials for anterior cruciate ligament reconstruction screws: The effect of glass surface functionalization on mechanical properties and cell behaviors, Materials Science and Engineering: C 77 (2017) 978-989.
J. Esmaeilzadeh, S. Hesaraki, M.H. Ebrahimzadeh, G.H. Asghari, A.R. Kachooei, Creep behavior of biodegradable triple-component nanocomposites based on PLA/PCL/bioactive glass for ACL interference screws, Archives of Bone and Joint Surgery 7(6) (2019) 531.
T. Niemelä, M. Kellomäki, Bioactive glass and biodegradable polymer composites, Bioactive Glasses, Elsevier2011, pp. 227-245.
V.V. Meretoja, T. Tirri, M. Malin, J.V. Seppälä, T.O. Närhi, Ectopic bone formation in and soft-tissue response to P (CL/DLLA)/bioactive glass composite scaffolds, Clinical oral implants research 25(2) (2014) 159-164.
H.O. Mayr, R. Hube, A. Bernstein, A.B. Seibt, W. Hein, R. von Eisenhart-Rothe, Beta-tricalcium phosphate plugs for press-fit fixation in ACL reconstruction-a mechanical analysis in bovine bone, The Knee 14(3) (2007) 239-244.
Y. Arama, L.J. Salmon, K. Sri-Ram, J. Linklater, J.P. Roe, L.A. Pinczewski, Bioabsorbable versus titanium screws in anterior cruciate ligament reconstruction using hamstring autograft: a prospective, blinded, randomized controlled trial with 5-year follow-up, The American journal of sports medicine 43(8) (2015) 1893-1901.
M.C. Park, J.E. Tibone, False magnetic resonance imaging persistence of a biodegradable anterior cruciate ligament interference screw with chronic inflammation after 4 years in vivo, Arthroscopy: The Journal of Arthroscopic & Related Surgery 22(8) (2006) 911. e1-911. e4.
W.H. Warden, D. Chooljian, D.W. Jackson, Ten-year magnetic resonance imaging follow-up of bioabsorbable poly-L-lactic acid interference screws after anterior cruciate ligament reconstruction, Arthroscopy: The Journal of Arthroscopic & Related Surgery 24(3) (2008) 370. e1-370. e3.
G. Schmidmaier, K. Baehr, S. Mohr, M. Kretschmar, S. Beck, B. Wildemann, Biodegradable polylactide membranes for bone defect coverage: biocompatibility testing, radiological and histological evaluation in a sheep model, Clinical oral implants research 17(4) (2006) 439-444.
R. Kontio, P. Ruuttila, L. Lindroos, R. Suuronen, A. Salo, C. Lindqvist, I. Virtanen, Y.T. Konttinen, Biodegradable polydioxanone and poly (l/d) lactide im-plants: an experimental study on peri-implant tissue response, International journal of oral and maxillofacial surgery 34(7) (2005) 766-776.
E. Waris, N. Ashammakhi, M. Lehtimäki, R.-M. Tulamo, M. Kellomäki, P. Törmälä, Y.T. Konttinen, The use of biodegradable scaffold as an alternative to silicone implant arthroplasty for small joint reconstruction: an experimental study in minipigs, Biomaterials 29(6) (2008) 683-691.
M. Barbeck, T. Serra, P. Booms, S. Stojanovic, S. Najman, E. Engel, R. Sader, C.J. Kirkpatrick, M. Navarro, S. Ghanaati, Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3D-printed scaffolds combining PLA and biphasic PLA/bioglass components–Guidance of the inflammatory response as basis for osteochondral regeneration, Bioactive materials 2(4) (2017) 208-223.
Z. Guo, D. Bo, Y. He, X. Luo, H. Li, Degradation properties of chitosan microspheres/poly (L-lactic acid) composite in vitro and in vivo, Carbohydrate polymers 193 (2018) 1-8.
T.T. Nguyen, T. Hoang, A.S. Ho, S.H. Nguyen, T.T.T. Nguyen, T.N. Pham, T.P. Nguyen, T.L.H. Nguyen, M.T.D. Thi, In vitro and in vivo tests of PLA/d-HAp nano-composite, Advances in Natural Sciences: Nanoscience and Nanotechnology 8(4) (2017) 045013.
C.B. Danoux, D. Barbieri, H. Yuan, J.D. de Bruijn, C.A. van Blitterswijk, P. Habibovic, In vitro and in vivo bioactivity assessment of a polylactic ac-id/hydroxyapatite composite for bone regeneration, Biomatter 4(1) (2014) e27664.
J. Esmaeilzadeh, S. Hesaraki, S.M.-M. Hadavi, M. Esfandeh, M.H. Ebrahimzadeh, Microstructure and mechanical properties of biodegradable poly (D/L) lactic acid/polycaprolactone blends processed from the solvent-evaporation technique, Materials Science and Engineering: C 71 (2017) 807-819.
H.A. Krebs, Chemical composition of blood plasma and serum, Annual review of biochemistry 19(1) (1950) 409-430.
W. Zhang, X.F. Walboomers, T.H. van Kuppevelt, W.F. Daamen, Z. Bian, J.A. Jansen, The performance of human dental pulp stem cells on different three-dimensional scaffold materials, Biomaterials 27(33) (2006) 5658-5668.
Z.-Y. Zhang, S.-H. Teoh, M.S. Chong, E.S. Lee, L.-G. Tan, C.N. Mattar, N.M. Fisk, M. Choolani, J. Chan, Neo-vascularization and bone formation mediated by fetal mesenchymal stem cell tissue-engineered bone grafts in critical-size femoral defects, Biomaterials 31(4) (2010) 608-620.
J. Van Meerloo, G.J. Kaspers, J. Cloos, Cell sensitivity assays: the MTT assay, Cancer cell culture: methods and protocols (2011) 237-245.
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 JCC Research Group

This work is licensed under a Creative Commons Attribution 4.0 International License.

